Aktuelle Projekte / Current Projects:
• Sustainable control of onchocerciasis today and tomorrow (SCOOTT, EU-INCO-Dev, 6.FP)
[1]
• Enhanced protective immunity against filariases (EPIAF, EC-Health, 7.FP)
[2]
• Gene expression analyses in resistant and permissive mouse strains following infection with the filarial nematode
[3]
• Impact of parasite infection on parasite- and allergen-specific immune responses
[4]
• Polyparasitism and its effect on specific immunity
[5]
• Echinococcus multilocularis infection-associated immune response
[6]
• Interdisciplinary Cooperation and Pragmatic Approaches for Control of Neglected Tropical Infectious Diseases
[7]
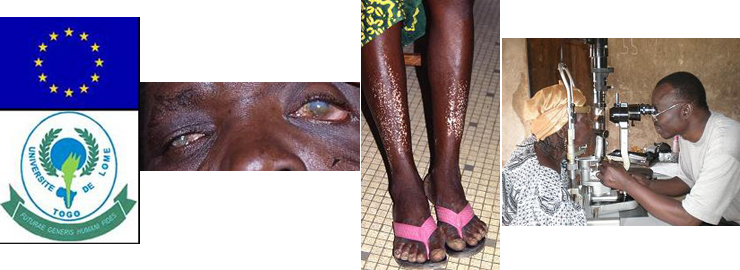 Sustainable control of onchocerciasis today and tomorrow
Sustainable control of onchocerciasis today and tomorrow
(SCOOTT, EU-INCO-Dev, 6.FP)
Meba Banla, Abram Agossou, Wolfgang Hoffmann, Peter Soboslay
Onchocerciasis, caused by the filarial nematode
Onchocerca volvulus, is the second most common infectious blinding disease of the developing world
afflicting 18 million people, 95% of who live in West and Central Africa. An estimated 500,000 persons are visually impaired and another 270,000 are
blind. Onchocerciasis is also characterised by severe and debilitating skin disease that has major economic and social consequences that include poor
school performance; low productivity, low income, and higher health related costs among infected adults; and extreme forms of social stigmatisation and
exclusion, especially among women.
Large areas of West Africa once endemic for onchocerciasis are now free of infections thanks to the work of the Onchocerciasis Control Program (OCP),
using vector control, and now the African Program for Onchocerciasis Control (APOC), relying primarily on mass treatment with ivermectin. Concomitant with
decrease in morbidity, there has been interruption of transmission.
However, in northern Benin,Togo, western Ghana and Guinea, areas covered by the APOC, transmission of
O. volvulus, re-infection and recrudescence of
disease has occurred. For sustained control of onchocerciasis it is critical to determine why the disease has reappeared. Some obvious explanations suggest
that 1) re-invasion of infected blackflies from areas where control has not been implemented has occurred; and 2) that resistance to ivermectin treatment
by
O. volvulus has developed.
The purpose of this project is to improve sustainable control of onchocerciasis (river blindness) through refinement of existing chemotherapeutic regimes and
identification of new targets and approaches for integrated control that will combine chemotherapy with vaccination. Today, the recommended control option
against onchocerciasis is repeated ivermectin treatment, which will have to be implemented for decades, and it remains unknown how repeated ivermectin therapy
might affect immunity against
O. volvulus in long-term. We study
O. volvulus-specific antibody reactivity and cellular cytokine production in onchocerciasis
patients receiving ivermectin (150ug/kg) annually for 16 years.
We find that long-term ivermectin therapy of onchocerciasis may not suffice to fully re-establish a balanced Th1 and Th2 immune responsiveness in
O.volvulusmicrofilariae-negative individuals. Such deficient reconstitution of immune competence may be due to an as yet continuing and uncontrolled re-infection
with
O. volvulus, but also, parasite co-infections can bias and may prevent the development of such immunity.
Litomosoides sigmodontis is the only filarial parasite that produces patent infection in laboratory mice and the pattern in infection and migration of
developing parasites mimics that are seen in human onchocerciasis, and like
O. volvulus, it harbours endosymbiontic
Wolbachia spp. Detailed knowledge of
immune responses in susceptible and resistant strains of inbred mice has been obtained. Furthermore, mice can be protected from challenge by vaccination
with irradiated L3 and the precise mechanism of protection has been established and found to be identical to those seen with
O. volvulus.
This project will adress and analyse if ivermectin treatment during ongoing infection in mice leads to protection or enhanced infection loads in secondary
infection and to identify the immunological correlates. Recent studies of cattle onchocerciasis suggest that ivermectin treatment may facilitate re-infection.
The possibility that ivermectin treated individuals may be more susceptible to re-infection in areas where control has stopped or has been interrupted, has
major consequences for the design and implementation of control programmes, including those that may involve vaccination in the future. This issue will be
investigated through treatment re-infection studies using the
L. sigmodontis-mouse model.
Visit the SCOOTT Website...
http://filaria.eu/
back to top next project
 Enhanced protective immunity against filariases
Enhanced protective immunity against filariases
(EPIAF, EC-Health, 7.FP)
The EPIAF Research Consortium
Vaccination is the most cost-effective means of disease control. The rationale for the feasibility of vaccination against filarial parasites
including onchocerciasis (river blindness, Onchocerca volvulus) and lymphatic filariasis (Wuchereria bancrofti or Brugia malayi) is founded on
two pillars.
First, there is evidence for immunity in hyper-endemic areas where a proportion of individuals live for many years with constant exposure
to infection yet never present with either clinical or parasitological signs of infection. These individuals in onchocerciasis endemic areas
are described as putative immune; while in the case of lymphatic filariasis, there are two distinct subgroups, non-infected individuals known
as endemic normals, and individuals with only latent infection, i.e. presence of adult worms (a marker being circulating filarial antigen, CFA)
but absence of microfilariae in the peripheral blood.
Second, successful immunisation of animal models against experimental and natural challenge has been achieved through vaccination with irradiated
infective third-stage larvae (L3) thus providing a powerful proof of principle that is also supported by work with the microfilarial stages of
these parasites, where animals acquire latent infection only and thus resemble the respective group of humans. Indeed, 100% protection against
microfilariae can be achieved in experimental models.
Identification of the mechanisms of protective immunity is a critical step in vaccine development. Studies of onchocerciasis and lymphatic
filariasis patients, and animal models have identified Th2 responses as playing a major role in protective immunity directed against invading
L3 and the microfilariae larvae.
To make theory a reality, this project aims to apply bioinformatics and computational models of immunity to the analysis of detailed clinical
and biological data from filarial infections to define protective immunity and identify vaccine candidates The study plan is designed to define
and characterise host/parasite interactions at the molecular level (the interactome) and in relation to development of protective immunity. It
will use high throughput microarray technologies to measure cytokine and other immunological responses of filarial patients with defined clinical
and parasitological presentations, and animals that express a demonstrable protective immunity.
Specific associations and interactions between filarial proteins and markers of protective immunity will identify potential vaccine candidates
that will be tested in Litomosoides sigmodontis/mouse model of filariasis that exhibits a demonstrable protective immunity. Confirmation of
immunological correlates of protection in humans will be determined by cross-sectional studies and retrospective analysis of data collected in
earlier epidemiological and immunological investigations.
back to top previous project next project
 Gene expression analyses in resistant and permissive mouse strains following infection with the filarial
nematode Litomosoides sigmodontis
Gene expression analyses in resistant and permissive mouse strains following infection with the filarial
nematode Litomosoides sigmodontis
Kirsten Bucher, Clemens Unger, Ute Kuntz, Wolfgang Hoffmann
Filarial infections such as onchocerciasis and lymphatic filariasis are caused by parasitic worms that belong to a particular group of tissue-invading
nematodes (Filarioidea). These infections may lead to severe morbidity (e.g. river blindness and elephantiasis) and are responsible for considerable
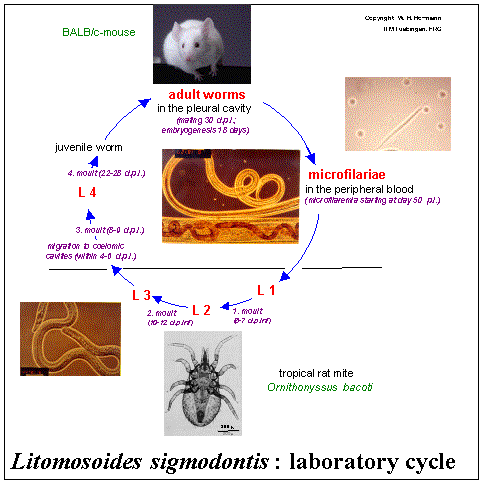
socio-economic problems throughout the tropics. Litomosoides sigmodontis is the only filaria known to undergo complete development in
mice (see figure below).
BALB/c mice are susceptible while mice of the C57BL/6 or DBA/1 strain are resistant. In patently infected BALB/c mice, and as seen in human filarial
infections, a Th2-type cytokine response predominates, initiating pathology as well as self-limiting the parasite load. Using a serial backcross strategy
with DBA/1 and BALB/c mice as parental strains we obtained congenic mouse strains sharing the BALB/c genetic background but which differ profoundly in their
susceptibility to microfilariae (1st larval stage of the filarial life-cycle).
As a major objective of this subproject genes and gene products that define the differences in susceptibility will be identified by two
approaches: 1. Scanning of the genomes of congenic mice and of the original parental strains by use of microsatellite and/or SNP analyses will
define the role of single host genes (in collaboration with the Max-Delbrueck-Center, Berlin). 2. Gene expression using microarray analyses will
be compared between resistant and permissive mouse strains.
The biological significance of selected candidate genes will be further characterized in vitro and in vivo. For these purposes the kinetics of
gene expression as well as the role of gene products will be investigated by quantitative RT-PCR and immunological assays, resp. In addition, infection
studies in appropriate knockout models might help to unravel the determinants for resistance and susceptibility in filariasis.
www.science.ngfn.de/6_196.htm
back to top previous project next project
 Impact of parasite infection on parasite- and allergen-specific immune responses
Impact of parasite infection on parasite- and allergen-specific immune responses
Jana Hegewald, Chris Lechner, Abram Agossou, Peter Soboslay
During pregnancy, transplacental migration of entire parasites or antigens from the mother into the fetus has been reported for many parasite species.
Such in utero exposure to parasites, has been recognized as a risk factor for children which may not only be extremely harmful for the developing child,
but prenatal contact with pathogens and allergens may also divert the normal immune maturation of the fetus. Prenatal contact with parasites and their a
ntigens may bias the development of immune competence, increase susceptibilty to infection, minder the postnatal success of vaccination, and prenatal
sensibilization may predispose for higher parasite densities and influence disease manifestation in later life.
The objectives of our work are to determine the effects and impact of maternal parasite infections (Filariasis, Malaria, Amebiasis and helminth
infections) during pregnancy on immune responsiveness in their children. We analyse in neonates their parasite-specific gene expression profiles and
specific cellular immune responses, their capacity to respond to secondary infectious challenge, and their predisposition for allergic disorders. We
compare gene expression and immune reactivity between newborns from infected African and exposure-free European mothers, and also, study the impact of
maternal parasite co-infection during pregnancy on prenatal immune sensitization. Furthermore, we want to know whether preventive and therapeutic control
measures during pregnancy (i.e. anti-malarial prophylaxis and anti-helminth treatment) will influence the extent and expression of prenatal
immune sensitization.
All our activities are based on solid collaboration between the Institute for Tropical Medicine in Tübingen, the Medical Faculty at University
of Lomé/Togo and the Primary Health Care Programs for the Central and the Urban Regions of Togo in Sokodé and Lomé.
back to top previous project next project
 Polyparasitism and its effect on specific immunity
Polyparasitism and its effect on specific immunity
David Hamm, Abram Agossou, Meba Banla, Stefan Geiger, Peter Soboslay
In rural West Africa, large populations remain chronically infected with intestinal helminth and protozoan parasites, and concurrently, schistosomiases
and filariases are often diagnosed as well. At least one third of the rural population remains chronically infected with more than one parasite and
triple and quadruplicate parasite infections are found in up to 20% of the population following thorough and careful diagnosis. Polyparasitism will
cause major overlaps in the symptoms presented, as well as multiple disease processes (
The Global Network for Neglected Tropical Diseases Control, GNNTDC).
Our study examined the impact of concurrent parasite infections (amoebiasis, filariasis, necatoriasis) and the effect of anti-parasite treatmenton
cytokine and chemokine responses in singly and poly-parasitized patients. Cytokine production and proliferation of peripheral blood mononuclear cells
were investigated in response to parasite- and bacteria-derived antigens, and mitogens. Cellular reactivity and parasite-specific Th1- and Th2-type
cytokine and chemokine profiles were investigated before and six weeks after treatment.
In those patients infected with three parasite species, cellular secretion of interleukin 5 (IL-5) and IL-12p40 by PBMC was strongly
diminished (p < 0.005) but IL-10 was elevated in parasiteinfected patients (p < 0.0001) in response to protozoa- and helminth-specific
as well as bacteria-specific antigens. Macrophage inflammatory chemokines (MIP-1a/CCL3 and MIP-1b/CCL4), macrophage-derived chemokine (MDC/CCL22)
and neutrophil activating chemokine (IL-8/CXCL8) were produced by PBMC in similar amounts in endemic controls and singly and poly-parasitized
patients, but thymus and activation regulated chemokine (TARC/CCL17) was produced the highest by PBMC from patients with triple parasite
infections (p < 0.0001). Following anti-parasite therapy, secretion of IL-12p40 and IL-5 augmented significantly in treated patients while
IL-10, MDC, MIP-1a, TARC and IL-8 substantially diminished (all p < 0.0001) when their PBMC were activated with parasite- and bacteria-specific antigens.
In summary, poly-parasitized patients responded to protozoa- and helminth-specific antigens with a compromised IL-5 and IL-12p40 but high IL-10 and a
substantial chemokine release. Chemokines may attract and activate effector cells in peri-parasitic tissues to limit parasite proliferation and dissemination,
while depressed IL-5 and IL-12p40 but prominent IL-10 may prevent eosinophil and cytotoxic cell-mediated inflammatory processes and pathogenesis to the host.
These changes following anti-parasite therapy disclosed the dynamics of an immune adaptation associated with parasite accumulation and also with clearance of
parasite infections.
back to top previous project next project
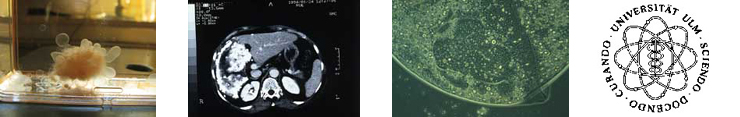
Echinococcus multilocularis infection-associated immune response
Chris Lechner, Beate Gruener (University Ulm), Peter Kern (University Ulm), Peter Soboslay
In humans infected with Echinococccus multilocularis clinical manifestations will develop following growth and proliferation of the larval metacestode
of E. multilocularis, and in the infected organs and tissues, mostly the liver, periparasitic cellular infiltrates will cause granolomatous lesions, necrosis,
host tissue destruction and ultimately organ failure.
Cellular immunity is considered the key defence against metacestode growth and dissemination, to which cytokine and chemokine responses in the periparasitic
granuloma may decisively contribute, for either progression or regression of parasite lesions.
In E.multilocularis endemic areas, only a few patients manifest with alveolar echinococcosis, and thus, defence mechanisms in the human host following
exposure and infection may limit parasite persistence and development of disease.
For progression to evident clinical manifestation, the E.multilocularis metacestodes have to be able to proliferate and such asexual growth is
initiated by exogenous budding of parasite vesicles which will progressively infiltrate the surrounding host tissue. Vesicles may contain protoscolexes
and by this means E.multilocularis will disseminate into other organs. For an effective limitation and containment of the developing metacestode in human
tissues, orchestrated parasite-toxic immune mechanisms have to be generated, however, such activation should not induce immune pathology and host tissue
destruction.
We have addressed in vitro the potential of E.multilocularis antigens to generate pro-inflammatory cytokines and chemokine responses in echinococcosis
patients and normal control individuals.
In patients, pro-inflammatory cytokines IL-1b and TNF-a responses were reduced and such selective diminution of immune mediators of inflammation may
prevent inflammatory pathology, while peripheral blood cells from patients were strong producers of IL-8 by which cellular attraction and effecter
cells migration into infected sites will be induced.
This opposite dynamics of inflammatory cytokines and chemokine release may prevent overwhelming and pathogenic inflammation and constitute an appropriate
response for attraction of effecter cells into the periparasitic tissues with the capacity to limit E.multilocularis metacestode proliferation, growth and
dissemination.
back to top previous project next project

Interdisciplinary Cooperation and Pragmatic Approaches for Control of Neglected Tropical Infectious Diseases
Université de Lomé, Faculté Mixte de Médecine et Pharmacie;
Service de Psychologie Pathologique, Clinique et Medicale, Togo;
AfrikaFonds, Health Care and Research, Steingaden, Germany;
University of Tübingen, Institute for Tropical Medicine, Germany;
Coopération Technique Allemande gtz-EPOS-PADESS-Togo
Richard Gantin, Meba Banla, Abram Agossou, Essoh Ayimba,
Peter Stingl, John Douti, Gnansa Djassoa, Peter Soboslay
Prioritization in tropical disease research and the focussing on three major health threats (HIV/TB/malaria) has particularly limited and neglected
the control and applied research in tropical infectious diseases of the poor. Scourges since millennia, schistosomiasis, soil transmitted helminth
infections, filariasis, trypanosomiasis, dracunculiasis and leishmaniasis account for 90% of the global disease burden, but less than 10% of the
global spending is allocated for their control. During the last few years however, the impact of neglected infectious diseases and their importance
to human health and development has been recognized to some greater extent.
Today, a few of the neglected tropical infectious diseases (NTID) are candidates for successful eradication (e.g. dracunculiasis). Some others
are announced close to elimination as a public health problem (e.g. onchocerciasis) and even though effective drugs are available for most NTID,
their successful control has been hampered not only by the re-allocation of priorities and resources for research and development but also by
economic and social crises in the endemic countries. As such, transmission and the recrudescence of NTID have been observed repeatedly in formerly
infection-freed areas, notably, in dracunculiasis, schistosomiasis, trypanosomiasis and onchocerciasis.
Still, the prospects for control, elimination or even eradication of some of the NTID are good, as they often have only a limited or focal
geographic distribution and seasonal transmission, effective drugs are available and successful containment of NTID has been demonstrated in
several countries. In the last decades, the re-emergence of presumably controlled diseases has shown that a regular, close, locally adapted and
sustained surveillance of the endemic population is needed in order to detect as early as possible those individuals, groups and endemic
populations re-exposed and infected. For control and disease surveillance, the possibility to detect new exposure and also to differentiate
chronic from expiring infections is especially important in areas which have previously been know as endemic, then declared as infection-free, but
today may be re-infested again.
Pragmatic approaches for successful control of NTIDs despite limited resources
The principal interventions are active case finding and treatment, in combination with monitoring, notably long term follow up of endemic
populations and epidemiological surveillance of the local situation, e.g. intensity of infection and parasite transmission. In situations
where limited budgets and resources restrict the application of advanced methodologies, e.g. serum or plasma-based measures of antibody
reactivity, circulating antigen or parasite DNA detection - the alternative collection of tear fluids, sputum, urines and stools is well
accepted during epidemiological surveys and such approaches are pursued for application.
Regular re-examination of the endemic population and of high risk groups by field-adapted and robust methodologies enables to identify new
or re-emerging endemic foci. Interdisciplinary co-operation of medical with social sciences, public and private partnership and personal
commitments are strategies not only for specific development and qualification of human resources, but also for better application of
effective control measures.
These strategies are implemented by regional and district-based technical platforms, reference points and laboratories for surveillance of
parasitoses such as dracunculiasis, onchocerciasis, lymphatic filariasis, schistosomiasis and soil transmitted helminths.
 |
|
 |
| Health education... |
|
.....also in difficult settings.... |
 |
 |
 |
| ...school-based intervention... |
...and monitoring and surveillance... |
...of parasite infections! |
| |
|
|
back to top previous project
















